We all know about the famous ozone layer that has been damaged for decades and whose holes are causing the melting of the poles according to specialists. What is this gas that protects us on one side but harms us on the other? What really is ozone pollution? Where does it come from? How are we impacted?
Ozone: a gas with two faces
At very high altitudes, in the stratosphere (10 to 60 km from ground), ozone filters and protects us from ultraviolet rays (ozone layer). Without ozone, there would be no life on earth.
At low altitude, in the troposphere (0 to 10 km high), ozone is a pollutant. Its highly oxidizing nature makes it harmful to health and vegetation. However, it is the same chemical compound that is both vital and harmful.
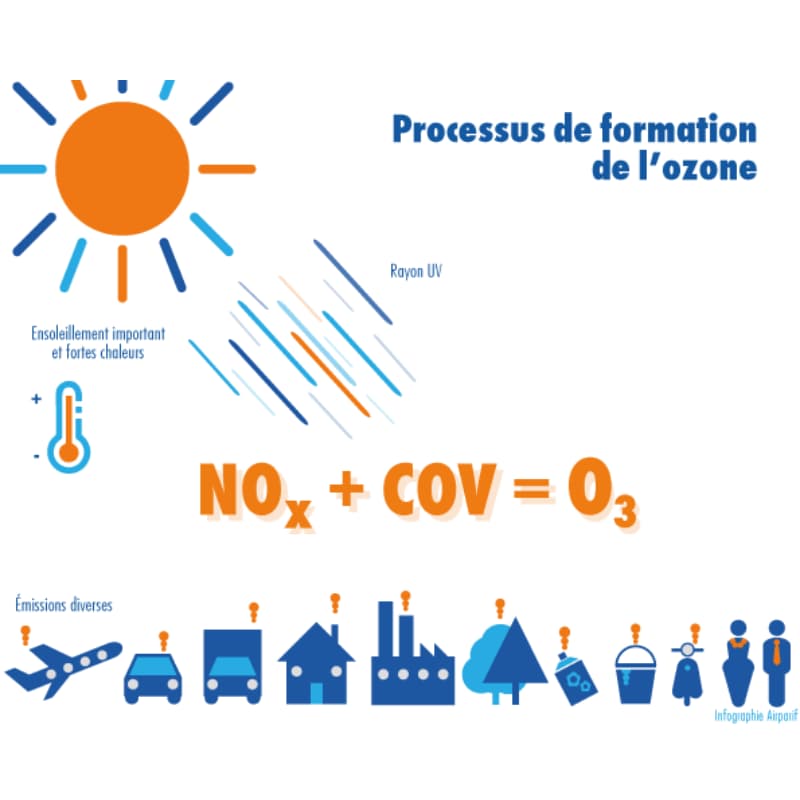
Tropospheric ozone (O3) is not directly released by a pollution source, which is why it is referred as a secondary pollutant . It is neither present in vehicle exhaust gases nor in factory fumes. It results from chemical reactions initiated by solar radiation, from so-called "precursor" pollutants such as nitrogen oxides (NOx) and Volatile Organic Compounds (VOCs). These come from human activities (vehicle exhaust gases, storage of petroleum products, use and manufacture of solvents or paints, etc.). Some species of trees also emit VOCs which play a role in the formation of ozone.
Due to its mode of formation, ozone is mainly present from June to August, with peaks appearing during the day between 1 p.m. and 7 p.m.

Where is ozone pollution highest?
Ozone levels may be higher in rural areas than in cities.
In urban areas or along roads, when ozone comes into contact with nitrogen oxides emitted by exhaust gases, a new chemical reaction occurs during which it is "consumed".
In high altitude or rural areas, ozone is mainly formed from urban pollution, as air masses can travel over great distances. Since precursor pollutants are in small quantities (notably nitrogen oxides), they cannot reduce ozone concentrations. This results in almost stationary ozone levels. In addition, among the precursor pollutants necessary for the formation of ozone is methane (CH4). This compound is much more present in rural areas than in cities, as it is emitted largely by agricultural activities, livestock and flora. Volatile organic compounds also promote the formation of ozone. They are partly of natural origin, produced by trees. The intensity of UV radiation has a strong influence on the formation of ozone. However, the higher the altitude, the greater the intensity of UV rays, hence the sometimes high concentrations measured in the mountains in summer.
What are the health effects of ozone?
Tropospheric ozone has an impact on health. It is an oxidizing gas, aggressive for the ocular and respiratory mucous membranes and which easily penetrates to the finest respiratory tracts. Exposure to ozone is likely to cause in the short term the occurrence of respiratory problems such as dry cough, triggering of asthma attacks, reduction of lung function.
Air pollution amplifies pollen allergies. Furthermore, during the summer period, the presence of ozone can be combined with the presence of pollens (especially grasses and ragweed) in the air. However, ozone increases the allergenic potential of pollen grains while weakening the respiratory tract. Allergic symptoms due to pollen can therefore be exacerbated for the most fragile people in the presence of ozone.
A strong impact on global warming
Ozone also has an impact on global warming. Ozone has the ability to absorb UV solar radiation in the upper layers of the atmosphere, thus allowing life on Earth. However, ozone also absorbs infrared (IR) rays that reach the ground, thus participating in the warming of the atmosphere through the greenhouse effect mechanism.
Tropospheric ozone is therefore both an atmospheric pollutant and the 3rd greenhouse gas on a global scale (after CO2 and methane) given its warming potential. But ozone is also subject to global warming, its formation being dependent on the temperature and UV energy of the Sun. According to a study conducted by the National Institute for Industrial Environment and Risks (INERIS) for the European Environment Agency (EEA), "climate change will have a penalizing effect on ozone pollution for a large part of continental Europe. It notes a forecast increase in ozone concentrations in summer of around 2 to 3 micrograms per m3 on average over the study period."
How to protect from a pollution peak?
In addition to avoiding using thermal engine vehicles and limiting physical activities, it is advisable to wear an anti-pollution mask such as Frogmasks.
With its FFP2/N95 filter which filters a minimum of 94% of particles down to 0.4µm, a Frogmask protects you in particular when traveling by bike or motorbike in the middle of road traffic.